- Home
- Fda Eua Ivd
FDA EUA IVD: Trusted Manufacturer of Diagnostic Tests for Supply and Wholesale Export from China
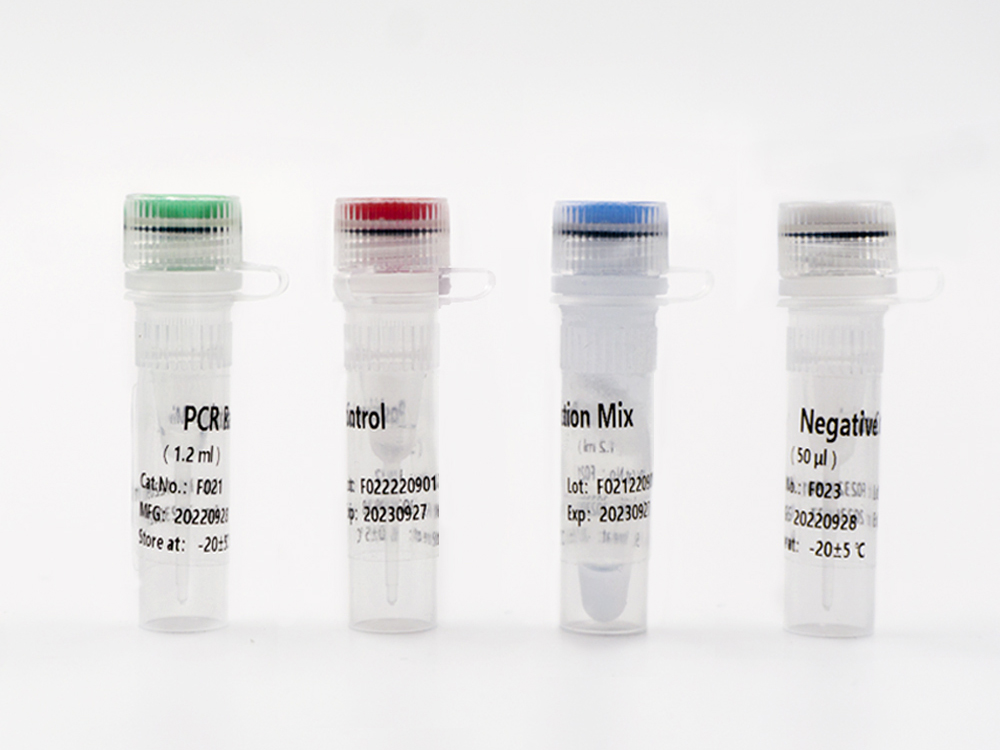
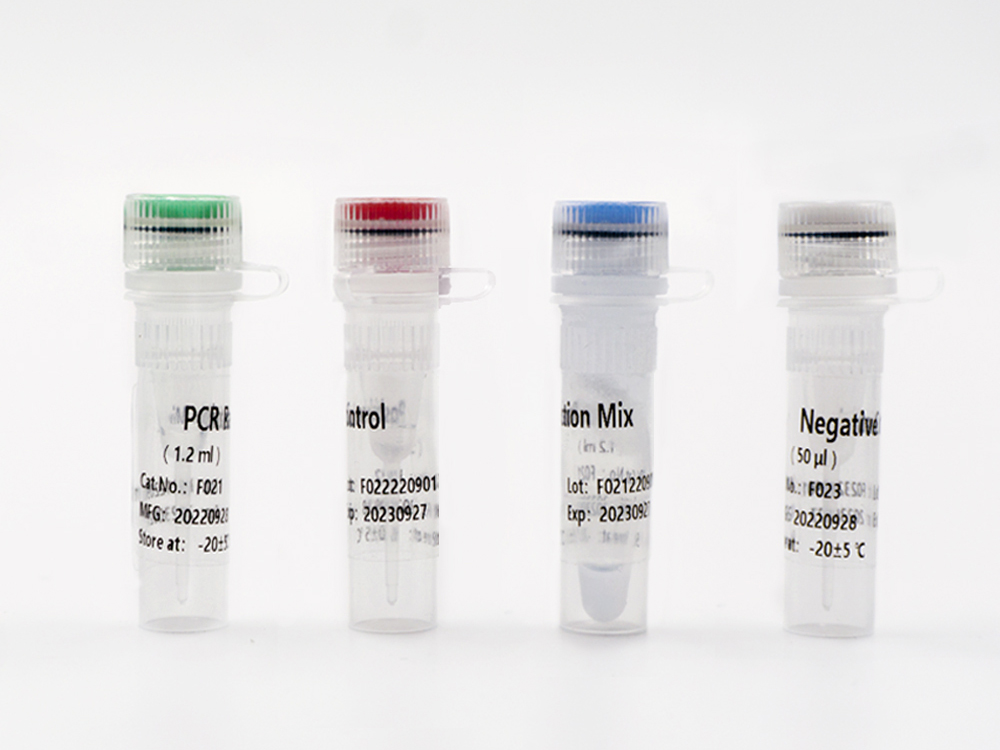
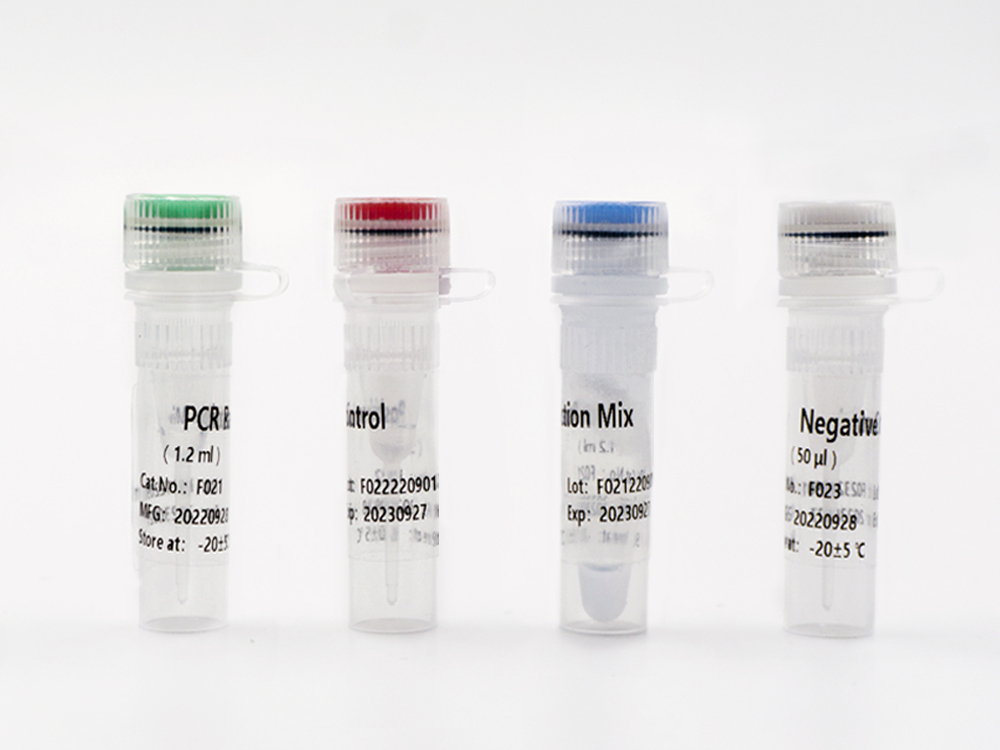
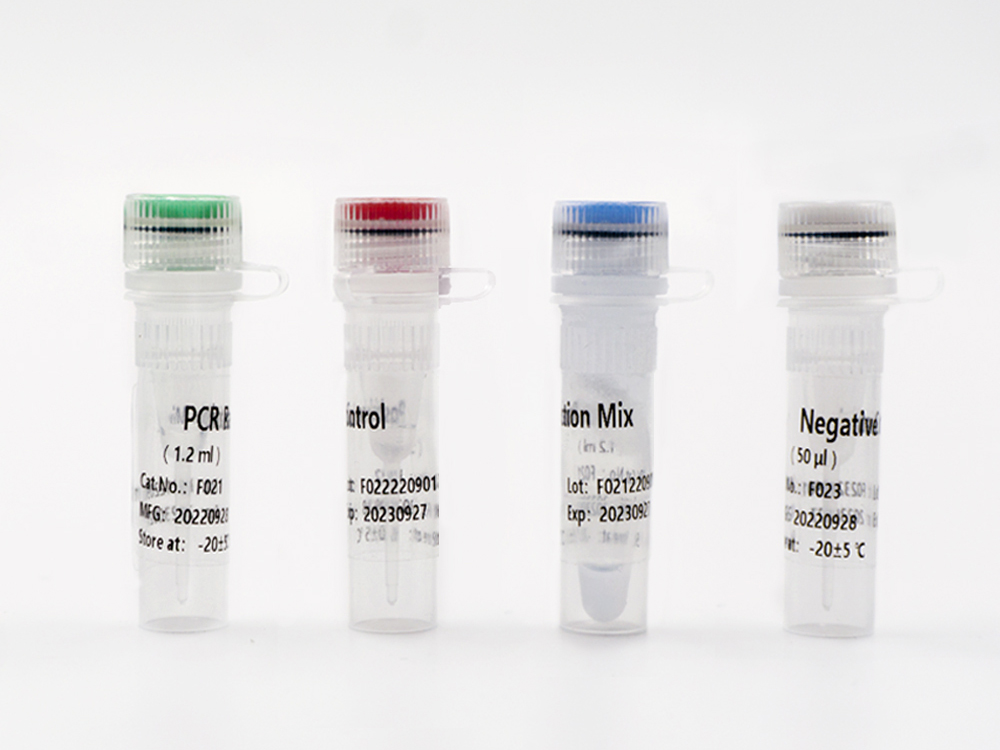
Shanghai Epiprobe Biotechnology Co., Ltd. is a leading manufacturer and supplier of in-vitro diagnostic (IVD) products in China. The company is proud to announce its latest product that has recently received Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA).
This new IVD is a highly accurate and reliable testing kit that has been designed to detect specific biomarkers in patient samples. The FDA EUA approval allows this product to be used urgently in the diagnosis of COVID-19. It is suitable for clinical laboratories and healthcare facilities seeking fast and reliable results.
As a trusted and experienced IVD manufacturer, Shanghai Epiprobe Biotechnology Co., Ltd. has invested heavily in research and development to ensure that all its products meet the highest global standards. With this latest FDA EUA approved IVD product, the company has again demonstrated its commitment to providing innovative and reliable products that make a positive impact on healthcare worldwide.
Shanghai Epiprobe Biotechnology Co., Ltd.

Company News
Related News
TAGMe DNA Methylation Detection Kits (qPCR) for Urothelial Cancer
Buy TAGMe DNA Methylation Detection Kits (qPCR) for Urothelial Cancer from our factory. Accurate and reliable results for early diagnosis. Shop now!
Gargle Nucleic Acid Extraction Reagents
Shop high-quality Gargle Nucleic Acid Extraction Reagents at our factory. We ensure efficient, reliable and affordable solutions for your scientific needs.
Nucleic Acid Extraction Kit (A01)
Shop our Nucleic Acid Extraction Kit (A01) from our factory. We provide high-quality products for efficient DNA and RNA extraction. Order now!
TAGMe DNA Methylation Detection Kits(qPCR) for Cervical Cancer
Shop TAGMe DNA Methylation Detection Kits for Cervical Cancer at our factory. High-quality and affordable solutions for accurate detection. Order now!
TAGMe DNA Methylation Detection Kits (qPCR) for Endometrial Cancer
TAGMe DNA Methylation Detection Kits (qPCR) for Endometrial Cancer - Factory-direct products for accurate and reliable detection of DNA methylation in endometrial cancer.
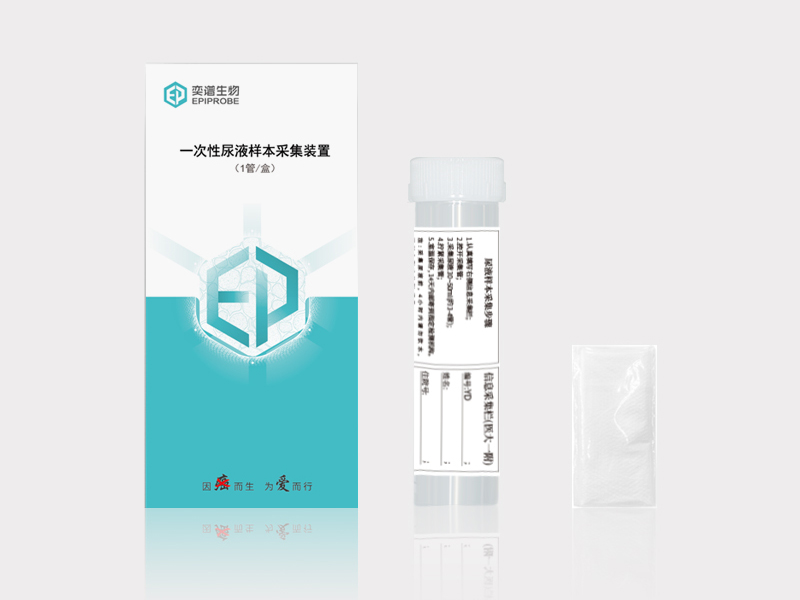
Disposable Urine Collection Tube
Looking for a reliable Disposable Urine Collection Tube? Look no further! We are a factory specializing in manufacturing high-quality urine collection tubes.
TAGMe DNA Methylation Detection for Pan-cancer
Shop now for TAGMe DNA Methylation Detection for Pan-cancer! Our factory delivers cutting-edge technology for early detection and monitoring of multiple cancer types.
TAGMe DNA Methylation Detection Kits (qPCR) for Cervical Cancer / Endometrial Cancer
Discover our TAGMe DNA Methylation Detection Kits (qPCR) for Cervical and Endometrial Cancer. Made in our factory, ensuring accurate and reliable results.
Nucleic Acid Extraction Kit (A02)
Shop our Nucleic Acid Extraction Kit (A02) at the factory-direct price. As a leading manufacturer, we provide high-quality products for efficient DNA/RNA extraction.
- Reviews
- Related Videos
Contact us
Please feel free to give your inquiry in the form below We will reply you in 24 hours